The Inreda AP® for healthcare professionals
The Inreda AP® is a treatment for people with type 1 diabetes that focuses on relieving the burden, both for the people with type 1 diabetes but also the healthcare professional.
Inreda AP® a unique system

Bi-hormonal
The Inreda AP® uses two hormones: insulin and glucagon, to prevent or treat hyper-/hypoglycaemia, respectively. The addition of glucagon makes the treatment unique in its kind.

Fully closed loop
The blood glucose levels are regulated fully automatic. People who use the Inreda AP® do not need to control or influence the regulation. Meal or exercise announcement is therefore not required. The Inreda AP® is thus a fully closed loop system.

All-in-one
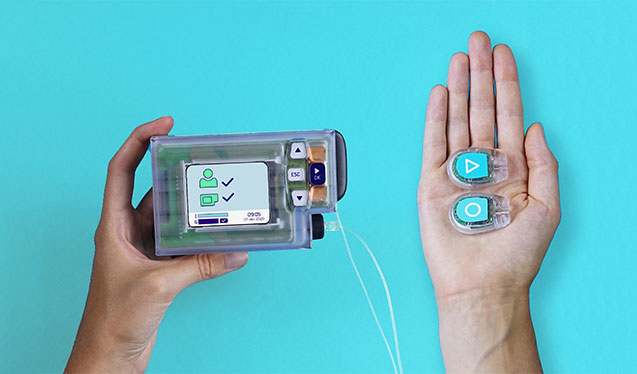
Two glucose sensors with a transmitter measure the glucose levels and physical activity. The levels are send wirelessly to the Inreda AP®. Based on this information, insulin or glucagon will be injected automatically. All components communicate with each other and are integrated into one system.
Responsive and self-learning algorithm
The algorithm of the Inreda AP® is responsive and reacts to changing blood glucose levels. Besides the responsiveness, the algorithm is self-learning. Insulin settings will be adjusted automatically to suit individual patients’ needs. Because of this, the glucose regulation will be optimized and personalized as well.
The Inreda AP® proves itself in a long-term study
After 1 year of bi-hormonal fully closed loop treatment…
- improves the Time In Range from 55.5% to 80.3%
- … improves the Time Below Range from 2.31% to 1.36%
- … improves the diabetes burden (assessed with the PAID questionnaire) from 30.0 to 10.0
- … achieves 98.6% of the patients the recommend treatment goal of >70% TIR (this was 22.7% of the patients at baseline) … achieves 97.2% of the patients the combined recommended treatment goals of >70% and <4% TBR (this was 10.7% of the patients at baseline).


Experiences healthcare professionals
“We are seeing a nice improvement in this measure.”
Since 2021, we have been working together with the Slingeland Hospital in Doetinchem. Alexandra, internist, notices that in the conversations with AP® users that glucose regulation is discussed much less, because the AP® takes over all this.
“People state they feel much fitter.”
In cooperation with the ZGT in Almelo, the use of the AP® is being examined. Thomas, technical physician, noticed that AP® users are much less concerned with regulating their glucose levels and that AP® users are feeling much fitter.
MijnInreda
Remote care is becoming increasingly more important, which is why an environment is being built to make this possible. MijnInreda will be an online environment accessible to both healthcare professionals and Inreda AP® users. In the future, professionals will be able to use this online environment to view patient reports, participate in treatment-focused training sessions, and utilize the platform as an information hub.
Projects and clinical studies
Every day, people are at the centre of our focus. To help as many people as possible in the future, we work passionately and attentively on the further development of the Inreda AP®. We do this in collaboration with our partners in various projects and clinical studies. The following projects are currently being worked on:
- Menzis and CZ
Two insurers investing in the further development of the Inreda AP® by enabling a total of 125 individuals to use the treatment at home every day. These individuals have been using the Inreda AP® treatment for over 2 years now.
- DARE Study
The Dutch Healthcare Institute and ZonMw have provided ‘’Promising Healthcare’’ funding to UMC Utrecht for a study on the (cost)effectiveness of the Inreda AP® compared to current treatment. A total of 240 individuals will participate in the Randomized Controlled Trial (RCT) study, with 120 individuals allowed to use the Inreda AP® for 1 year. During this study, the Inreda AP® will be compared with other treatments such as pen and pump therapy.
Other clinical studies
The system is currently CE MDR certified (MDR certified) for ages 18 and above. To further increase availability, clinical studies are underway. Research has been conducted for adolescents (12 to 18 years old), and in the future, we aim to expand this research to include this age group in the use of the Inreda AP®. Additionally, we also collaborate with Amsterdam UMC to research the effectiveness of the Inreda AP® with individuals who have undergone pancreatectomy or pancreatitis, and therefore have type 3c diabetes.
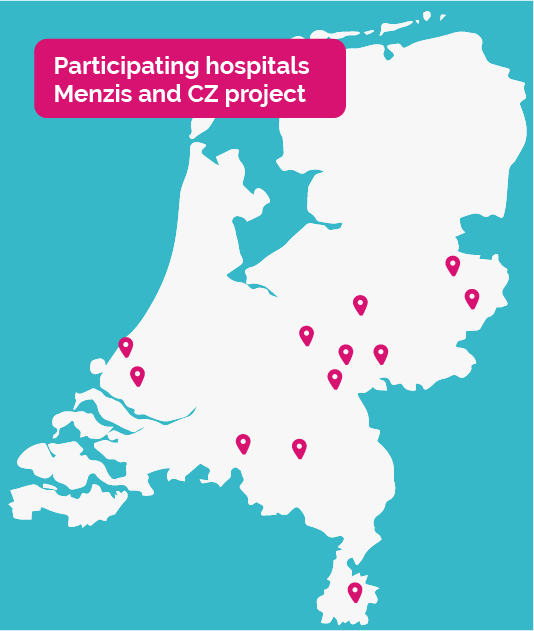
The following hospitals are participating in the Menzis and CZ project:
- Canisius Wilhelmina Ziekenhuis te Nijmegen
- Medisch Spectrum Twente te Enschede
- Rijnstate ziekenhuis te Arnhem
- Slingeland ziekenhuis te Doetinchem
- Ziekenhuis Gelderse Vallei te Ede
- Ziekenhuisgroep Twente Almelo te Almelo
- Deventer Ziekenhuis te Deventer
- Radboud UMC te Nijmegen
- Zuyderland Medisch Centrum te Heerlen
- Elisabeth TweeSteden Ziekenhuis te Tilburg
- Erasmus MC te Rotterdam
- Haga Ziekenhuis te Den Haag
- Elkerliek Ziekenhuis te Helmond
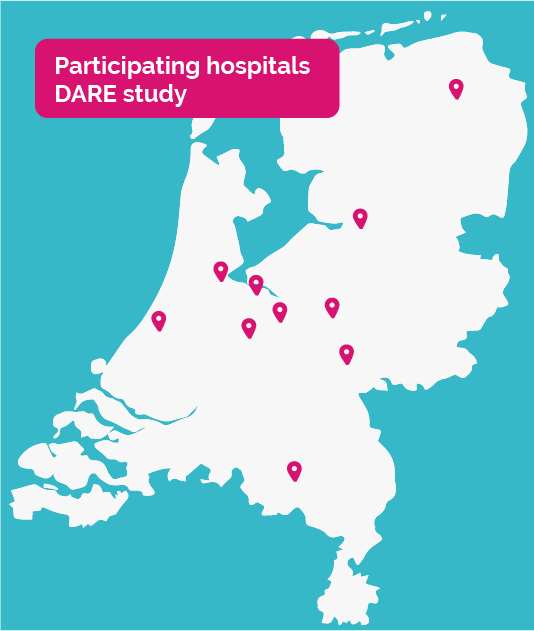
The following hospitals participate in the DARE study, coordinated by the UMC Utrecht:
- Catharina Ziekenhuis te Eindhoven
- Rijnstate ziekenhuis te Arnhem
- Amsterdam UMC , locatie VU
- Amsterdam UMC , locatie AMC
- OLVG te Amsterdam
- Martini Ziekenhuis te Groningen
- UMC Groningen
- Leids UMC te Leiden
- Tergooi ziekenhuis te Hilversum
- Meander Ziekenhuis te Amersfoort
- Gelre Ziekenhuis te Apeldoorn
- Isala te Zwolle
- St. Antonius Ziekenhuis te Nieuwegein.